- Founded
- 2023
- Employees*
- 1
- Funding to Date*
- $275,000
Vascular endothelial Growth factor, also known as VEGF, is primarily responsible for forming and maintaining new blood vessels. Under abnormal conditions, especially in the eye, such as age-related macular degeneration (AMD) and diabetic retinopathy, it causes the formation of abnormal blood vessels, which can bleed, leak, and eventually lead to scar formation and vision loss. Anti-VEGF therapy, which is administered by injection into the back of the eye, is the standard of care for severe retinal diseases, especially wet AMD. These therapeutics command a market over $12B today just for the eye alone, but those patents will soon expire, and pharma is scrambling to remix and extend their existing patents.
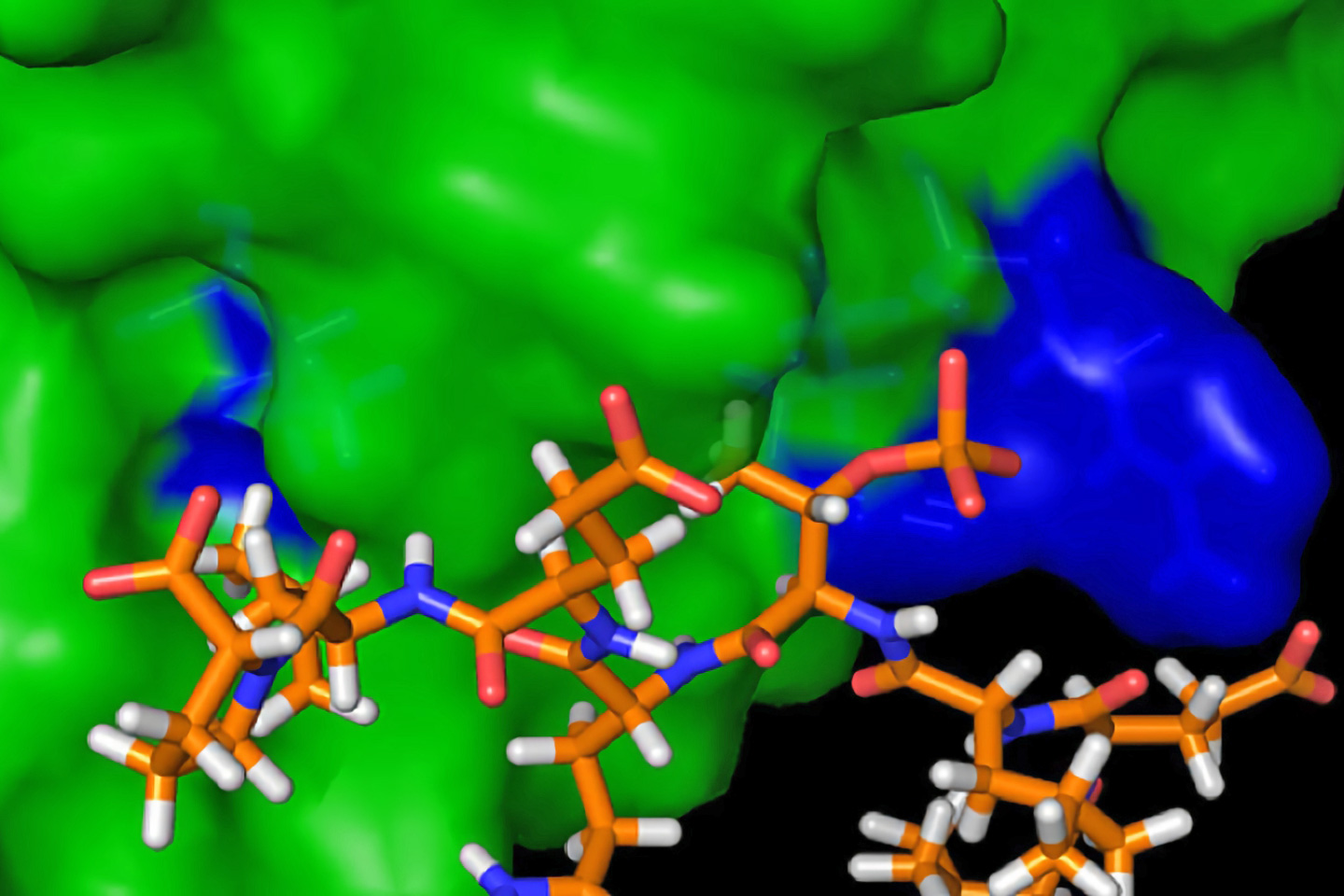
ViAn Therapeutics has patented a highly potent and stable anti-angiogenic peptide that is already found in our body. The peptide is so small compared to the standard-of-care antibodies, it can be developed as a simple topical eye drop for ophthalmology indications. Not only will this enable a larger segment of AMD patients to be treated much earlier in the disease (and thereby avoid progression to severe disease), this opens up a vast market opportunity in mild to moderate diabetic retinopathies and other retinal vein occlusive diseases where a topical treatment will be highly preferred over retinal injections. ViAn Tx has already demonstrated high penetrance into the back of the eye as an topical formulation, and are putting together a pre-IND package now to further prove its safety and efficacy in rabbits.